Medical device electronics and software that are FDA‑compliant and secure from day one.
Build fast without compromising quality or compliance. Powered by medical device veterans, world-class cybersecurity experts, and AI‑assisted development.
Explore our medical device software services, learn why teams choose Bold Type, and meet our engineering leadership. Dive into expert insights on the resources hub.
"For sophisticated systems that include embedded software, mobile applications, and cloud, Bold Type's expertise in cybersecurity is truly unparalleled in the industry. They've been a trusted partner for years and I couldn't recommend them more highly."Michael Fong
"Bold Type has been a trusted product development partner since our earliest days. They led the development of the Leva® Pelvic Health System, and continue to work seamlessly with our internal team to improve our app based on patient feedback."Jim O'Connor
"Bold Type has been a trusted partner of our product development team for several years. We have valued their technical expertise, ability to help us troubleshoot issues and solve problems. Their QMS system and attention to detail in building out DHFs has been robust."Shayna Massi
Leading industry experts in wireless, cloud-connected medical devices.
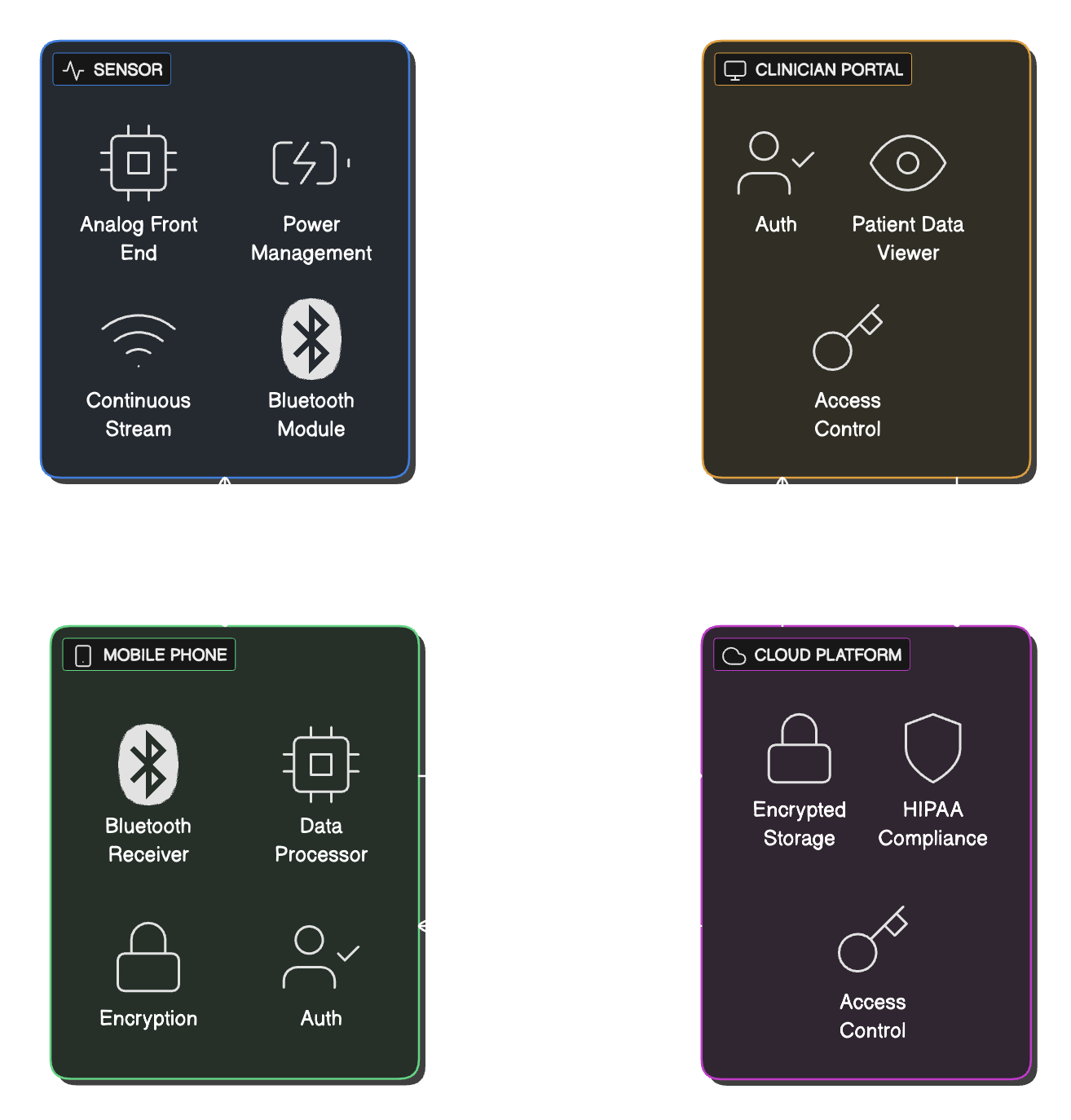
Medtech startups don't get second chances
Launch with technical, regulatory, and timeline confidence
Compliance gaps that derail FDA approval
One missing document or security vulnerability can set back your 510(k) submission by months. Our ISO 13485-certified process ensures nothing falls through the cracks.
Delays that jeopardize fundraising or market entry
Time-to-market is critical. Our AI-powered development and proven frameworks help you ship faster without cutting corners on quality or compliance.
Cost overruns and scope creep
Unclear requirements lead to budget disasters. We provide fixed-scope engagements with transparent pricing and regular milestone reviews.
Partners who don't "get" medical device complexity
Generic software developers don't understand FDA regulations or medical device workflows. We speak your language and know your challenges.
Bold Type: U.S.-based, ISO‑certified experts delivering polished, compliant, and fast software.
Deep FDA regulatory fluency and documentation experience
Our documentation follows standards like IEC 62304, IEC 62366, IEC 60601, ISO 14971, SW 96, and dozens of FDA guidance documents.
World‑class cybersecurity leadership via our CyberMed division
Our cybersecurity division, CyberMed, provides expert threat modeling, penetration testing, and post-market cybersecurity management.
Integrated team (firmware, wireless, mobile, cloud, testing)
One team handles your entire technology stack. No coordination headaches between multiple vendors or communication gaps.
Efficiency driven by foundation code and AI coding agents
We use proprietary code blocks and secure AI to accelerate development. No "vibe coding" here; just experts using the latest tools for safe efficiency.
How It Works: Our 3-Step Plan
From initial consultation to market-ready product
Schedule a Discovery Call
We'll discuss your product goals, timeline, and regulatory requirements. No sales pressure; just honest advice about your path to market.
Get a Tailored Roadmap
Get a detailed project plan with milestones, deliverables, and compliance checkpoints. Know exactly what to expect and when.
Build with Confidence
Execute your roadmap with regular reviews and continuous compliance validation. Ship secure, compliant, and polished software.
Ready to launch with confidence?
Join the medical device startups who choose Bold Type for faster, safer, compliant development.