FDA-Ready Mobile Apps for Medical Devices
We develop secure iOS and Android apps that seamlessly interface with wireless medical devices. Built for patients, clinicians, and R&D teams, our cross-platform solutions reduce development time by 50% while ensuring HIPAA compliance and FDA readiness from day one.
Key Features
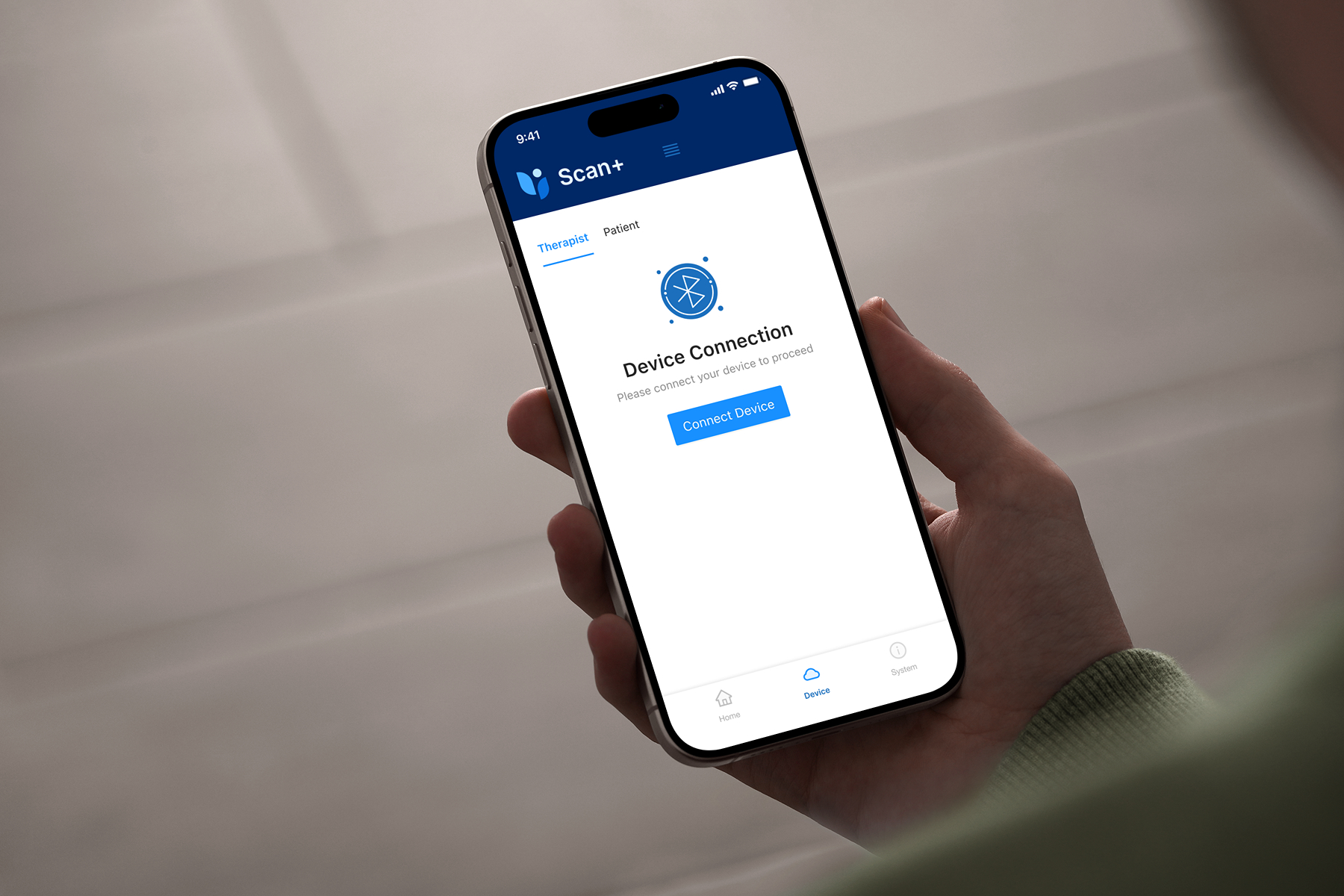
Secure BLE Communication
Robust Bluetooth Low Energy communication with medical devices, implementing secure pairing, data encryption, and reliable connection management.
- Custom GATT service integration
- Secure pairing and bonding protocols
- Connection state management and retry logic
- Background communication support
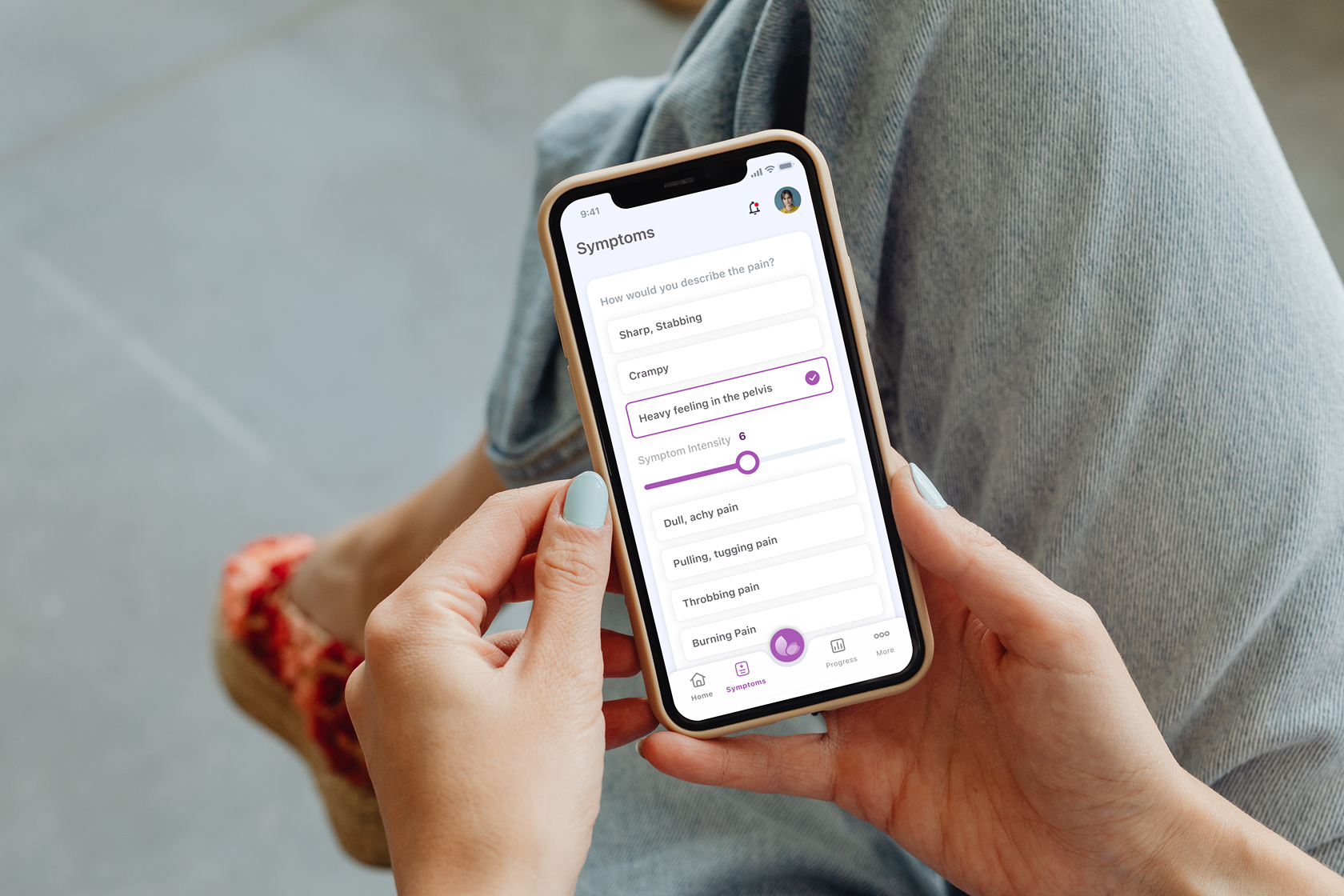
HIPAA-Aligned User Data Handling
Comprehensive data protection and privacy controls designed to meet HIPAA requirements for handling protected health information (PHI).
- End-to-end encryption for PHI
- Secure authentication and authorization
- Audit logging and access controls
- Data retention and deletion policies
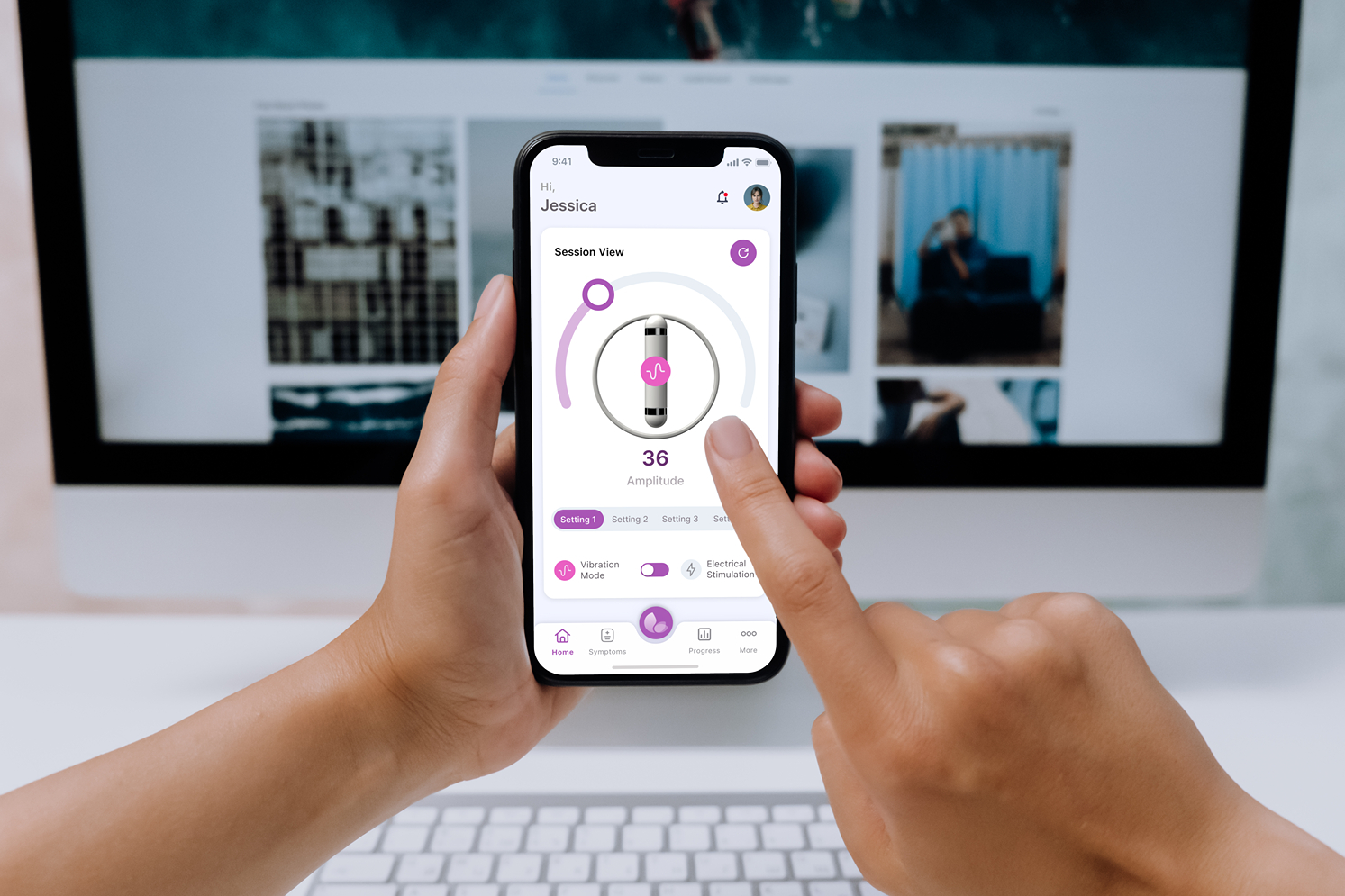
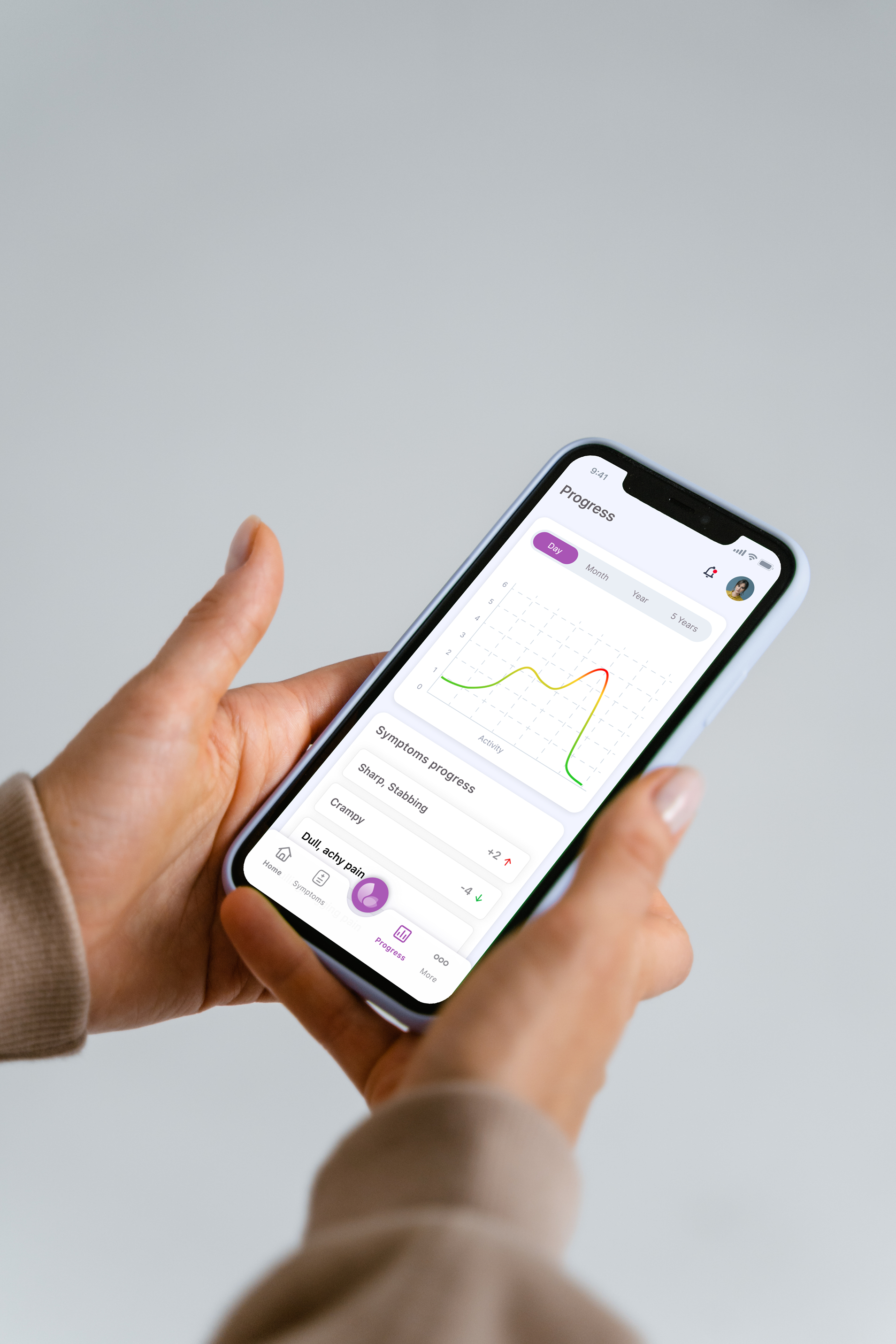
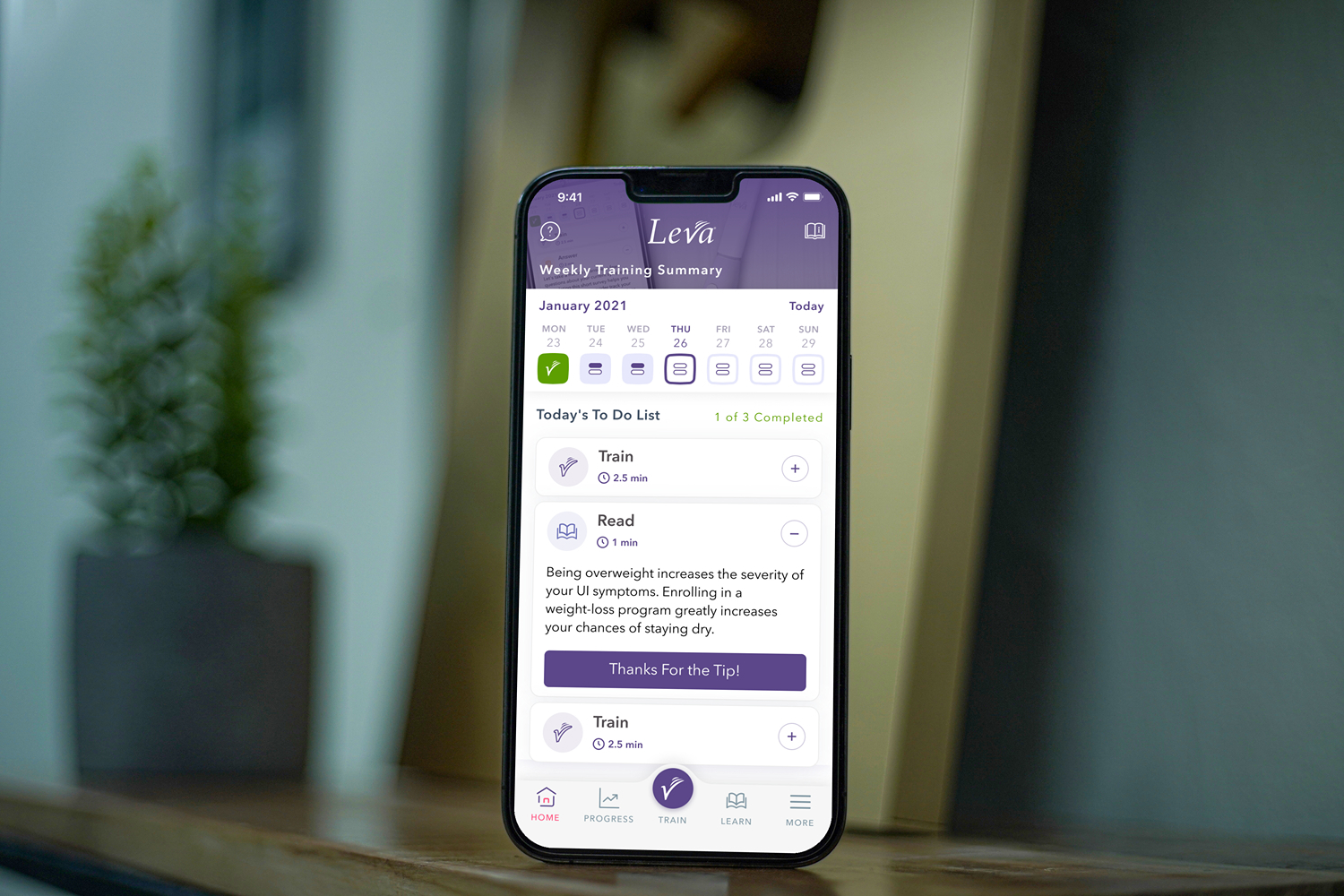
Real-time Device Telemetry and Feedback
Live data visualization and interactive feedback systems that provide users with immediate insights from their medical devices.
- Real-time data charts and graphs
- Interactive device controls and settings
- Push notifications and alerts
- Historical data trends and analysis
On-device Storage, Encryption, and Sync
Secure local data storage with encryption and cloud synchronization capabilities for seamless user experience across devices.
- Encrypted local database storage
- Offline functionality and data caching
- Cloud synchronization with conflict resolution
- Data backup and restore capabilities
Mobile Integration into Full System DHF
Complete integration of mobile applications into the medical device's Design History File (DHF) with proper documentation and regulatory compliance.
- Mobile app risk analysis and hazard identification
- Software requirements and design documentation
- Verification and validation testing protocols
- Traceability matrix and change control
Cross-Platform Development Advantage

One Codebase, Two Platforms
Using React Native and Flutter, we develop apps that work seamlessly on both iOS and Android from a single codebase. This approach reduces development time by 40-60% while maintaining native performance and user experience.
Faster Time to Market
Cross-platform development means faster iteration cycles, reduced testing overhead, and quicker deployment. Critical for medical device startups with tight timelines and limited resources.
Easier Maintenance
Bug fixes, feature updates, and regulatory changes only need to be implemented once and deployed to both platforms simultaneously, reducing long-term maintenance costs and complexity.
Mobile App Use Cases
Patient-Facing Apps
Intuitive interfaces for patients to monitor their health data, receive treatment guidance, and communicate with healthcare providers.
Clinician Tools
Professional-grade apps for healthcare providers to monitor multiple patients, review data trends, and make informed clinical decisions.
Internal Testing Tools
Development and QA tools for device testing, calibration, configuration, and troubleshooting during development and manufacturing.
Ready to Build Your Medical Device Mobile App?
Let's discuss your mobile app requirements and how we can create an intuitive, secure, and compliant app that enhances your medical device ecosystem.